News
Advances in kiwifruit postharvest knowledge
The paper by Shimeles Tilahun et al., fully downloadable, analyzes the convergence of physiological insights, omics, and nondestructive technologies for postharvest management of kiwifruit
Kiwifruit (Actinidia spp.) is valued for its sensory quality and nutritional richness but faces postharvest challenges such as rapid softening, chilling injury, and physiological disorders.
Conventional management strategies help maintain quality yet insufficient to capture the complexity of ripening, stress physiology, and cultivar-specific variation.
Recent research emphasizes the continuum from preharvest to postharvest, where orchard practices, harvest maturity, and handling conditions influence quality and storage potential.
Omics-driven studies, particularly transcriptomics and metabolomics, have revealed molecular networks regulating softening, sugar–acid balance, pigmentation, antioxidant properties, and chilling tolerance. Integrated multi-omics approaches identify key biomarkers and gene–metabolite relationships linked to ripening and stress responses.
Complementing omics, nondestructive estimation technologies, including hyperspectral imaging, near-infrared spectroscopy, acoustic profiling, and chemometric models are emerging as practical tools for real-time classification of maturity, quality, and storability.
When calibrated with omics-derived biomarkers, these technologies provide predictive, non-invasive assessments that can be deployed across the supply chain.
Together, the convergence of postharvest physiology, omics, and nondestructive sensing offers a pathway toward precision quality management and sustainable kiwifruit production.
This review synthesizes recent advances across these domains, highlighting mechanistic insights, practical applications, and future directions for integrating omics-informed strategies with commercial postharvest technologies.
Introduction
Kiwifruit (Actinidia spp.) has emerged as one of the most economically and nutritionally significant horticultural crops, widely cultivated across Asia, Europe, New Zealand (Oceania), and the Americas [1]. Its commercial success stems not only from unique sensory properties but also from its high concentrations of vitamin C, phenolics, flavonoids, carotenoids, and other health-promoting metabolites that make it a functional food [1,2].
However, maintaining fruit quality during long storage and global distribution presents formidable postharvest challenges, including chilling injury, softening, and pathogen susceptibility [3,4]. These physiological constraints have driven extensive research into optimizing harvest maturity, storage conditions, and handling practices [5,6,7,8].
Continuum from preharvest to postharvest management
Recent advances in postharvest research emphasize the continuum from preharvest to postharvest management, where field-level practices such as agro-ecological choice, chitosan or melatonin spraying, and harvest timing directly influence storability and consumer quality [4,7,9].
Postharvest technology
In addition, controlled atmosphere storage, temperature management, biopreservation strategies, particularly the application of beneficial microorganisms (e.g., antagonistic yeasts and bacteria) and natural elicitors that stimulate host defense responses, and chemical treatments such as 1-methylcyclopropene (1-MCP) and jasmonates (JAs) have been extensively investigated to mitigate postharvest softening, chilling sensitivity, and disease incidence [10,11,12].
Need of more nondestructive approaches - The omics
Despite such progress, the complex interplay of ripening, stress physiology, cultivar-specific traits, harvest maturity, and storage conditions cannot be fully addressed by conventional physicochemical indices alone, highlighting the need for omics-driven and nondestructive approaches to provide deeper mechanistic insights into postharvest management.
In this context, omics technologies (transcriptomics, metabolomics, proteomics, and their integration) provide transformative insights into the molecular and metabolic basis of fruit development, ripening, and stress responses [1,13,14].
Beyond broad conceptual advances, specific transcriptomic studies have shed light on gene regulatory networks that govern postharvest behavior.
For instance, investigations have identified genes and pathways linked to softening, sugar–acid metabolism, ethylene biosynthesis and signaling, antioxidant defense, and cultivar-dependent responses [15,16,17,18].
Metabolomic approaches
In parallel, metabolomic approaches complement these insights by profiling dynamic shifts in primary and secondary metabolites across cultivars, tissues, and developmental stages [19,20,21,22,23].
These metabolomic approaches also clarify dynamic responses during ripening and storage [2,16,24] and reveal how treatments such as bagging, chitosan, methyl jasmonate (MeJA), 1-MCP, ozone, blue light, and coatings reprogram sugar metabolism, cell wall disassembly, lipid turnover, and antioxidant defenses [17,25,26,27,28,29,30].
In addition, metabolomics has identified stress-tolerance markers, linking flavonoid metabolism, lipid peroxidation, and energy balance to differences in chilling or anaerobic tolerance [15,31,32,33], and has guided breeding by distinguishing germplasm and ploidy variants with superior profiles of anthocyanins, carotenoids, or vitamin C [20,34,35].
Multiomic integration
More recently, multi-omics integration has emerged as a transformative approach in kiwifruit research for detailed gene–metabolite networks, linking transcriptional regulators with metabolic pathways for sugars, flavonoids, anthocyanins, proanthocyanidins, and vitamin C, thereby clarifying how key transcription factors coordinate nutritional and sensory quality traits [16,21,35,36].
Multi-omics has also revealed how storage and treatment interventions such as calcium, chitosan coatings, blue light, ethanol fumigation, and riboflavin photosensitization modulate lipid metabolism, cell wall disassembly, energy pathways, and antioxidant defenses, offering mechanistic explanations for delayed ripening and enhanced disease resistance [27,29,37,38,39].
Beyond ripening and treatments, multi-omics has been applied to abiotic stress responses, such as freezing, chilling, and cold-chain fluctuations, where it identified metabolic markers like flavonoids, lipids, and organic acids, and regulatory hubs like HsfA3a that govern resilience or susceptibility across genotypes [15,31,40].
Importantly, integration across omics has provided novel biomarkers for harvest maturity and storability, exemplified by candidate transcripts and metabolites predictive of storage disorder [41], offering practical potential for supply-chain management.
Moreover, resources like the Kiwifruit PanGenome Database (KPGD) have expanded opportunities for trait characterization, enabling the integration of structural variants, SNPs, and transcriptomic datasets into multi-omics pipelines for comparative genomics and molecular breeding [42].
Although many studies remain correlative and cultivar-specific, the accumulating evidence highlights that multi-omics not only deepens mechanistic insight but also provides tangible tools for developing precision breeding, optimized storage, and eco-friendly postharvest strategies in kiwifruit.
Non destructive quality evaluation
Complementing these biological insights, nondestructive quality estimation technologies are transforming how fruit quality is monitored across the supply chain. Portable tools such as near-infrared (NIR) sensors, smartphone-based platforms, and LED–photodiode devices now enable rapid, low-cost, and real-time assessment of fruit maturity and internal quality, reducing reliance on destructive sampling [43,44].
Advances in imaging, spectroscopy, and machine learning have also significantly improved accuracy in detecting defects, predicting ripeness, and classifying textural properties under commercial conditions [45,46,47].
Recent studies demonstrate that combining multiple sensing modalities such as hyperspectral imaging (HIS) with Fourier transform near-infrared (FT-NIR) spectroscopy, fluorescence imaging with machine learning, or volatile analysis with HSI further enhances robustness and predictive power [48,49,50].
Collectively, these technologies are moving beyond research settings toward practical application, offering scalable, non-invasive solutions for growers, distributors, and retailers. When integrated with omics-derived biomarkers and mechanistic models, nondestructive sensing holds strong potential to support precision harvest decisions, optimize storage and transport, and deliver consistent fruit quality to consumers.
Taken together, the convergence of postharvest physiology and technology, omics, and nondestructive estimation provides an unprecedented opportunity to advance sustainable kiwifruit production, storage, and distribution.
The challenge, translating laboratory findings into commercial practices
Nevertheless, key challenges remain in translating laboratory findings into commercial practices, integrating multi-omics datasets with sensor platforms, and validating biomarkers across cultivars, environments, and seasons.
This review therefore synthesizes recent progress in kiwifruit postharvest physiology and technology, transcriptomics, metabolomics, integrated omics, and nondestructive quality estimation, with the goal of outlining mechanistic insights and practical strategies that can inform both breeding programs and industry adoption.
To ensure transparency and minimize selection bias, the review followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines. A structured literature search was conducted in Web of Science, Scopus, PubMed, and ScienceDirect for studies published between 2016 and 2025.
The search initially retrieved 180 records, of which 30 duplicates were removed. The remaining 150 articles were screened by title and abstract, and 130 were subjected to full-text evaluation. Ultimately, 126 peer-reviewed studies met the inclusion criteria and were incorporated into this review.
Eight earlier foundational works (2016–2019) were retained to provide historical and mechanistic context, while the majority of included studies (118) were published from 2020 onward, reflecting the rapid expansion of omics-driven research and nondestructive sensing technologies in kiwifruit.
This temporal distribution confirms that the evidence synthesized here is strongly anchored in the most recent decade of postharvest and molecular innovation.
Sources
Advances in Kiwifruit Postharvest Management: Convergence of Physiological Insights, Omics, and Nondestructive Technologies
Shimeles Tilahun, Min Woo Baek 2,4ORCID,Jung Min Baek 2,Han Ryul Choi 4,5,DoSu Park 1 andCheon Soon Jeong 2,4,*ORCID
https://www.mdpi.com/1467-3045/48/1/9
Curr. Issues Mol. Biol. 2026, 48(1), 9; https://doi.org/10.3390/cimb48010009 (registering DOI)
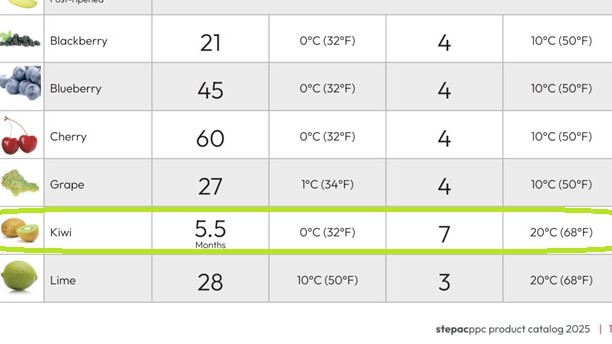
The picture belongs to StePac PPC catalogue, manufacturer of advanced modified atmosphere packaging solutions for fresh produce, and shows that the postharvest life can be extended from 5,5 to 7 months. The company Paclife also counts on packaging solutions to extend kiwifruit postharvest life.


.png)








