News
Fungal-bacterial alliances have a potential role in disease severity
Samir Droby et al. demonstrated that co-inoculation with certain bacterial isolates significantly increased lesion development compared to the fungus alone - The authors propose that future disease control strategies adopt a microbiome-driven perspective
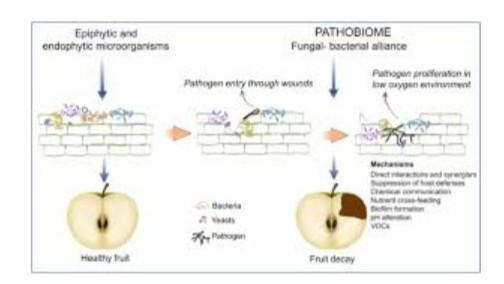
Postharvest losses caused by fungal pathogens are traditionally explained through a single-pathogen paradigm, where disease results from pathogen pathogenicity and virulence factors acting on a susceptible host under favorable conditions.
However, emerging evidence challenges this view, pointing instead to the role of microbial consortia - the postharvest pathobiome - in shaping infection outcomes.
In this opinion article, we explore the potential hidden alliance between fungi and bacteria that enhances the virulence of postharvest pathogens.
Using apple - P. expansum as a model system, preliminary experiments revealed that co-inoculation with certain bacterial isolates significantly increased lesion development compared to the fungus alone, whereas bacteria alone were non-pathogenic.
Amplicon sequencing further showed that fungal infection altered the wound microbiome, favoring the proliferation of anaerobic and facultative anaerobic bacteria.
We discuss multiple mechanisms by which bacteria may contribute to fungal virulence, including nutrient cross-feeding, biofilm formation, chemical signaling, pH modulation, alteration of host defenses, and the formation of endosymbiotic alliances.
Recognition of this fungal-bacterial synergy has profound implications for postharvest management, as fungicide or biocontrol-based strategies may overlook the supportive role of bacteria and inadvertently favor more resilient pathogenic consortia.
We propose that future disease control strategies adopt a microbiome-driven perspective, integrating high-throughput sequencing, metabolomics, imaging, and in silico modeling to disentangle these interactions and guide the development of sustainable, biologically informed interventions.
Understanding and manipulating such inter-kingdom alliances may represent a crucial step toward reducing postharvest decay and improving global food security.
Introduction
Fungal spoilage of produce is a major cause of postharvest losses and represents a significant portion of global food waste, posing a threat to both food security and economic stability (Avery et al., 2019, OECD-FAO Agricultural Outlook 2025-2034, 2025).
The dominant paradigm that has existed in postharvest pathology for decades is based on the premise that a single pathogen is the sole and primary cause of a specific disease, with well-documented examples of postharvest pathogens such as Penicillium spp., Botrytis cinerea, Alternaria spp., Monilinia spp., Colletotrichum spp., and others (Eckert and Ratnayake, 1983, Matrose et al., 2021, Cárdenas et al., 2025).
These major pathogens, particularly necrotrophic species that require injured host tissues for infection and are thus referred to as wound pathogens, cause decay by deploying an array of pathogenicity and virulence factors (Sacristán et al., 2021, Bi et al., 2023).
For example, Penicillium expansum, the causal agent of blue mold on apples, breaks down host tissues using a variety of cell wall-degrading enzymes (CWDEs), lowers the pH by releasing gluconic acid, and produces patulin, a mycotoxin associated with significant food safety concerns (Luciano-Rosario et al., 2020, Wang et al., 2023).
B. cinerea, is another major necrotroph that causes gray mold in various fruits and vegetables. It combines CWDEs and phytotoxic compounds to kill host cells and consume nutrients in decaying tissue (Bi et al., 2023).
According to the single pathogen/single disease paradigm, disease is the outcome of a susceptible host, optimal environmental conditions, and pathogenicity and virulence effectors that combine to initiate a successful infection (Petrasch et al., 2022, Prusky and Romanazzi, 2023, Prusky et al., 2025, Li et al., 2025).
An holistic view, based on the postharvest pathobiome concept
However, in recent years, this monocausal perspective of postharvest pathology is being increasingly challenged by a more nuanced and holistic understanding of microbial ecology and the central role of the microbiome in the health and disease of fresh produce after harvest (Kuruppu et al., 2024, Sui et al., 2024, Droby et al., 2025).
This new paradigm builds on the foundation of the 'postharvest pathobiome' concept (Bass et al., 2019, Droby et al., 2022, Ngolong Ngea et al., 2024), which posits that disease is not an isolated event caused by a single pathogen but rather the outcome of complex interactions between the host, the primary pathogen, the environment, and a wider community of microorganisms associated with the pathosystem.
In this relation, while research investigating microbial interactions in postharvest systems is not new, it has historically focused on the beneficial role of bacteria in antagonism and competition against fungal pathogens, aiming to find biocontrol agents.
The current frontier in plant-associated microbial ecology lies in identifying and characterizing the opposing phenomenon of synergistic and mutualistic interactions that is involved in pathogen virulence. Research investigating this concept as a basis for disease development, however, is still in its nascent stages, and remains to be investigated.
While anecdotal and specific case studies are accumulating, a comprehensive understanding of the underlying molecular mechanisms, the diversity of these inter-kingdom interactions across different host-pathogen systems, and their precise environmental triggers remains largely unexplored.
In this regard, the number of cases of bacterial/fungal pathogen synergies in plant pathology in general, and postharvest pathology in particular, has been limited. In contrast, bacteria involved in modulating fungal growth and virulence in human diseases, as well as bacteria that regulate fungal pathogenesis, have been reported in several pathologies (McAlpine et al., 2023).
A potential hidden alliance between fungal and bacterial species
This opinion article introduces and explores the concept of a potential hidden alliance between fungal and bacterial species that can enhance fungal pathogenicity and virulence in postharvest pathosystems.
It also highlights the need for further research to fully elucidate the complexity of the disease process in certain postharvest fungal-host interactions in fruits and vegetables.
We argue that overlooking this sophisticated inter-kingdom synergy, which extends from external interactions to intimate endosymbiotic relationships, limits the efficacy of current management strategies, especially those based on biological control, and necessitates a paradigm shift in our approach to postharvest disease control and research.
Source
A hidden alliance: The potential role of bacteria in the virulence of postharvest fungal pathogens
Samir Droby, V. Yeka Zhimo, Vijay Kumar Sharma, Rotem Bartuv, Michael Wisniewski, Hongyin Zhang, Shiri Freilich, Davide Spadaro
Postharvest Biology and Technology, Volume 234, April 2026, 114130